Those Boston Dynamics robots are getting less and less clunky, but they’re still firmly occupying the uncanny valley. Their latest, Spot Mini, is impressive, but when you see the one with an extensible manipulator…
The Discovery Channel (their reputation is so bad, you’re probably already booing) has a ‘reality’ show called Venom Hunters. It is about teams of courageous reptile experts who make a living — and save lives — by capturing rare and deadly venomous animals in the wild, and milking them of toxins for use in antivenoms. Sounds cool, doesn’t it? It was probably snapped right up by the channel when the premise was presented to them.
Only a few problems with it, though: they’re mostly not experts, that’s not how venom is collected, nobody makes a living off this fictitious profession, it’s unlikely that any of the venom is being used for its stated purpose, and at least some of the animal captures are staged, using captive snakes.
Over on Science Sushi, you can read a very detailed exposé of the phony staff, the bogus stories, and their potentially illegal activities. It’s as phony as that mermaid ‘documentary’.
Man, the Discovery Channel must really hate Christie Wilcox. She’s filleting them.
We at UMM are having our yearly HHMI-sponsored summer research program. In addition to having undergraduates working away in our labs, we also have some more social activities — and yesterday we joined with the science students at the Morris public schools for a bottle rocket launch.
No, not that kind of bottle rocket. In this case, they using two liter plastic bottles, filling them up with some water, and then pumping them up with air pressure. Then…whoosh, off they go. The rockets were also assembled with fins and nose cones and those traditional bits. The elementary and middle school kids had to deal with various constraints — they had “budgets”, and had to “pay” for every bit of cardboard and duct tape they stuck on their bottles, and also for the water fuel and the amount of pressure they put in the bottles. They also had specific goals: distance traveled, time aloft, that sort of thing. Our HHMI students had no constraints, so it was a little unfair. They weren’t part of the competition, though, and the kids whose rockets outperformed the college students’ got extra points on their victory, so it was OK.
The UMM rockets did pretty well, but yeah, some of the kids’ rockets with their minimal approach did do better.
I’m teaching our science writing course in the Fall, and I’m also one of the instructors in our teachers’ workshop next month (we still have room for more participants!). And now I’ve found a useful, general, basic paper that I have to hand out.
Motulsky, HJ (2014) Common Misconceptions about Data Analysis and Statistics. JPET 351(1):200-205.
What it’s got is clear, plain English; brevity; covers some ubiquitous errors; will be incredibly useful for our introductory biology students. You should read it, too, for background in basic statistical literacy. Here’s the abstract.
Ideally, any experienced investigator with the right tools should be able to reproduce a finding published in a peer-reviewed biomedical science journal. In fact, however, the reproducibility of a large percentage of published findings has been questioned. Undoubtedly, there are many reasons for this, but one reason may be that investigators fool themselves due to a poor understanding of statistical concepts. In particular, investigators often make these mistakes: 1) P-hacking, which is when you reanalyze a data set in many different ways, or perhaps reanalyze with additional replicates, until you get the result you want; 2) overemphasis on P values rather than on the actual size of the observed effect; 3) overuse of statistical hypothesis testing, and being seduced by the word “significant”; and 4) over-reliance on standard errors, which are often misunderstood.
I can probably open any biomedical journal and find papers that commit all four of those errors.
Not this again. CNN is running another article about “X causes cancer!”, where in this case X is coffee. Not regular coffee, just very hot coffee. That is, coffee served at a temperature high enough to cause painful burns might also increase the incidence of esophageal cancer.
Huh. OK. You know, living causes cancer. Epidemiological studies like the one cited are important for identifying possible problems, but your whole life is a great long exercise in risk management where you balance doing things against cowering in terror. We have to consider realistic assessment of risk. So I was going to actually read the study (the short summary given is that an analysis of a thousand studies found that “drinks consumed at very hot temperatures were linked to cancer of the esophagus in humans”, but no numbers were given), but CNN screwed up: their link to the study goes to a paper on the carcinogenicity of pesticides in the Lancet instead. I thought I’d rummage around and try to find it myself, but instead I found this editorial in the latest issue which was pretty good, much better than yet another study that finds a superficial cancer link. So I’m including the whole thing right here.
Is it just me or does that fish look horrified?
I’m working with a student this summer on a project to measure the dynamics of a migrating cell population, and in addition to making pretty pictures and collecting data, we’re going over papers in the scientific literature. One of those papers is about filopodia as sensors.
For those of you who don’t know your cellular anatomy, migrating cells have this kind amoeboid movement in which their cytoplasm oozes in a kind of bulk flow into expanding volumes of membrane, but they also may make long, spindly, delicate ‘antennae’ that reach out in multiple directions. These filamentous processes are called filopodia. Moving cells probe their environment by sending out these little scouts that can sense signals, either repulsive signals that tell the cell to not go that way, or attractive signals that can trigger the cell to flow in a particular direction.
The illustrations in this paper are kind of quirky, but nice. To show how cells respond to signals in the environment, they use an octopus as a stand-in for the cell, with its arms representing filopodia.
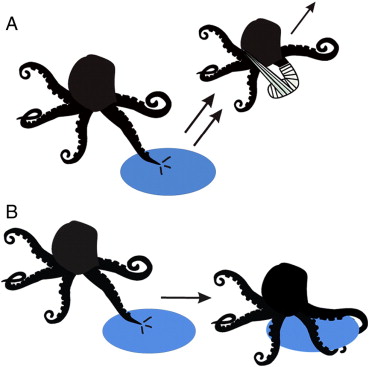
Repulsive and attractive interactions in axon pathfinding. A) Repulsive interaction. When the cell contacts the target, the forward momentum of the growth cone is halted. The movement of the veil/lamellipodium resumes at the right or left of the contact site. As a result, the growth cone turns aside. The formation and turnover of FCs within the filopodium are correlated with its behavior during this interaction. B) Attractive interaction. The cell contacts a stationary target and binds tightly to it.
Despite the blatant octopomorphization, something about this appeals to me. I’ve been describing these cells we’re watching as spidery, but clearly I’ve been using the wrong phylum as a metaphor.
I like the bandaged arm image. One of the things we’re sometimes seeing is that cells don’t just retract and limp away, sometimes they literally die and explode into little fragments. This needs an octopoid illustration.
