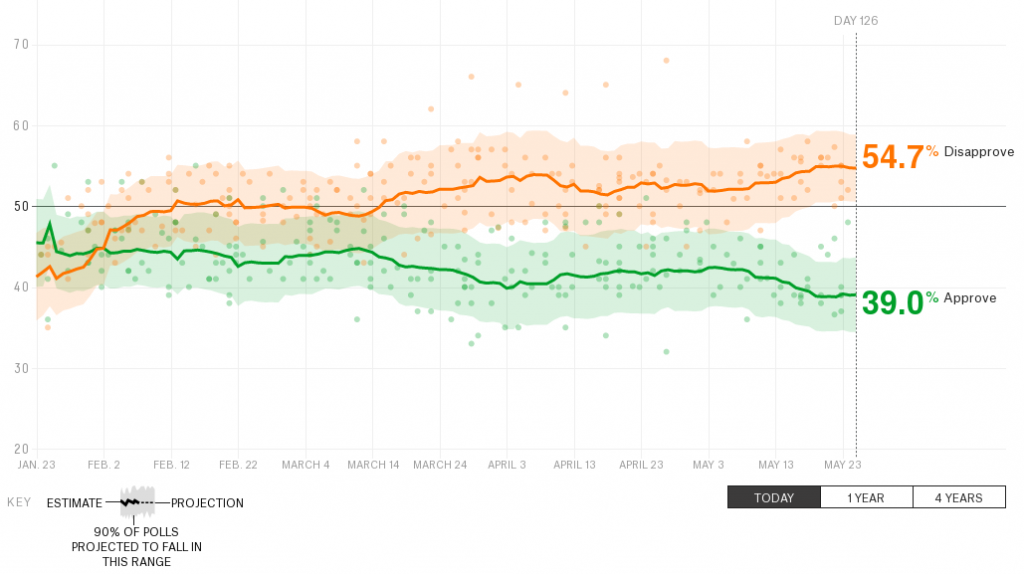
Last time, I pointed out that within the Boghossian/Lindsay kerfuffle no-one was explaining how you could falsify gender studies. As I’ve read more and more of those criticisms, I’ve noticed another omission: what the heck is in a gender studies journal? The original paper only makes sense if it closely mirrors what you’d find in a relevant journal.
So let’s abuse my academic access to pop open the cover of one such journal.
Gender & Society, the official journal of Sociologists for Women in Society, is a top-ranked journal in sociology and women’s studies and publishes less than 10% of all papers submitted to it. Articles analyze gender and gendered processes in interactions, organizations, societies, and global and transnational spaces. The journal publishes empirical articles, along with reviews of books.
They also happened to be at the top of one list of gender studies journals. I’ll go with their latest edition as of this typing, volume 31 issue 3, which is dated June 2017.